As someone who got #HepC via Vertical transmission, I don’t just have a lot of thoughts on the matter, I tend to make a lot of comments, public ones. The CDC’s new expansion incorporates almost all of the concerns I’ve voiced to the CDC, CDPH and others over the years when they solicit input from the community.
The latest MMWR from the CDC goes into The escalating prevalence of Hepatitis C Virus (HCV) infections, particularly among reproductive-aged and pregnant populations, underscores a burgeoning health challenge. The Centers for Disease Control and Prevention (CDC) revamped its guidelines in 2020 to encompass universal screening for adults and pregnant individuals, aiming to adeptly manage and curb the proliferation of this ailment.
Epidemiological Trends:
The surge in HCV infections since 2010 is alarming, with acute cases notably tripling among individuals aged 20-39. This uptick correlates with an increase in injection drug use, necessitating targeted interventions. The domino effect of escalating acute infections to chronic conditions emphasizes the urgency for early detection and management. (There’s likely to be a systems issue here, based on a lack of accessible mental health management options, and likely increases in self-medication, among a myriad of other co-occurring circumstances and conditions)

Transmission Dynamics:
Percutaneous exposure to infected blood, predominantly through injection drug use, is a significant transmission route. Perinatal transmission is another vector of concern, thus necessitating prudent strategies to mitigate HCV spread from mother to infant. Understanding these transmission dynamics is pivotal for devising effective preventive frameworks. But its important not to judge people, based on data, rather its imperative we support the person through their diagnosis to cure, as it helps reduce stigma and potential resulting trauma.
Testing, Diagnosis and why this change is important:
The CDC’s 2020 guidelines update underscored universal screening to foster early detection and management. The guidelines propose refined testing protocols for infants and children born to HCV-infected mothers, mirroring the advancements in HCV testing methodologies. This was made possible in part due to public comments from fantastic folks like yourself!
Clinical Progression of Perinatally Acquired HCV Infection:
The variance in clinical progression among perinatally infected children underscores the need for timely interventions. While some achieve spontaneous clearance, others advance to chronic infections, necessitating vigilant monitoring and management to avert severe liver disease. (That’s me, they’re talkin’ bout me ya’ll, End Stage Liver Disease by age 22)
The recent CDC guidelines expand on the diagnostic procedures for HCV infection in pregnant individuals and perinatally infected children. The shift from risk-based to universal screening for HCV during pregnancy aims to bridge the previous under-diagnosis gap. A notable uptick in HCV screening during pregnancy has been witnessed, and all professional societies now advocate for testing perinatally exposed infants for HCV, albeit with variations in the timing.
Clinical Management and Treatment of Perinatal HCV Infection highlights a 6%-7% transmission rate of HCV among perinatally exposed children, (this is almost twice as high as when I was diagnosed with HepC in 1999) with Direct-Acting Antiviral (DAA) therapy emerging as a viable treatment avenue for children aged ≥3 years. The CDC, along with other professional bodies like AASLD-IDSA and NASPGHAN, is steering towards maximizing early diagnosis and linkage to care through perinatal testing for HCV.
Great, but What are the CDC’s new testing recommendations for Hepatitis C Testing Among Perinatally Exposed Infants and Children?
Perinatally exposed infants should receive a NAT for HCV RNA at age 2–6 months to identify children in whom chronic HCV infection might develop if not treated. (Importantly it skips the Antibody test, because the test could indicate a false positive, capturing the maternal antibodies, TBH the antibody test, while a useful epidemiological tool, does beg the question as it to its usefulness for children at all, when, at least anecdotally, it causes a lot of confusion for patients.)
-Infants with detectable HCV RNA should be managed in consultation with a health care provider with expertise in pediatric hepatitis C management.
-Infants with an undetectable HCV RNA result do not require further follow-up unless clinically warranted.
-Infants and children aged 7–17 months who are perinatally exposed to HCV and have not previously been tested should receive a NAT for HCV RNA.
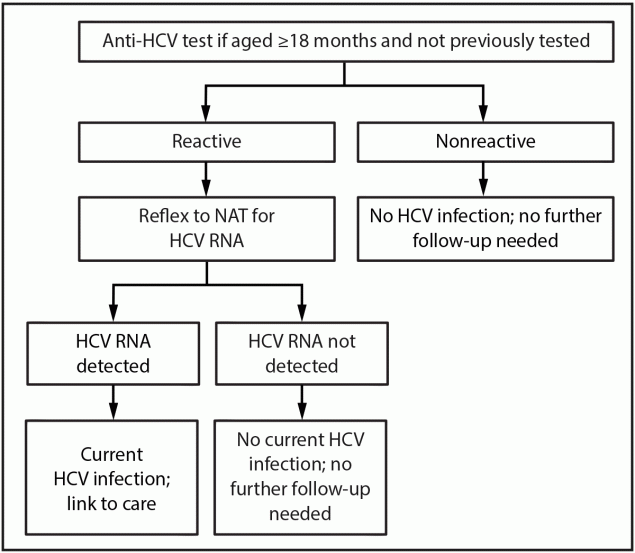
-Children aged ≥18 months who are perinatally exposed to HCV and have not previously been tested should receive an anti-HCV test with reflex to NAT for HCV RNA (Figure 4). (This is an interesting update because it helps with follow-up and appropriate diagnosis, it also shores up holes in terms of late diagnosis, like in my case, where my mom was diagnosed when I was 12, albeit because of my positive case. Although if someone has a negative NAT HCV RNA result, it would seem unnecessary to do an antibody test, which again, just reiterating the likely waste of time and resources of an antibody test.)
Cost-Effectiveness Considerations, The CDC conducted a novel analysis to evaluate the cost-effectiveness of perinatal Hepatitis C Virus (HCV) testing strategies, comparing the current method of testing at 18 months to a proposed strategy of testing at 2-6 months. The mathematical modeling study revealed that earlier testing yielded increased diagnoses, improved health outcomes, and was cost-saving at a population level, with savings of $469,671 per year. Universal screening strategies, although enhancing diagnoses and health outcomes, were found to be less cost-effective. The study, evaluated for quality via the CHEERS checklist, substantiated that testing known exposed infants at 2-6 months is both cost-effective and beneficial for better health outcomes.
Hepatitis C Elimination
Ongoing monitoring and potentially revising recommendations in line with evolving epidemiology and treatment modalities for hepatitis C during pregnancy and among children are on the CDC’s radar. The quest for more data on universal screening, prevalence rates among pregnant persons, and the natural history of perinatal hepatitis C is unending. The deployment of highly sensitive and specific NATs for RNA detection among perinatally exposed infants and children is a beacon of hope in identifying those with HCV infection. This initiative aligns with the broader agenda of national hepatitis C elimination goals, showcasing a concerted effort to ensure no population is left behind in this health crusade. The road towards hepatitis C elimination is laden with challenges, yet with cohesive strategies and relentless pursuit of early diagnosis and treatment, strides towards a healthier tomorrow are within grasp. As someone who understands the impact of a pediatric diagnosis of Hepatitis C, in terms of the emotional experience, and challenge with family interactions and stigma, this is a welcome update to capture potential cases that might otherwise be missed.
Get Tested, Get Treatment, Let’s End Hep C!
Panagiotakopoulos L, Sandul AL, et al. CDC Recommendations for Hepatitis C Testing Among Perinatally Exposed Infants and Children — United States, 2023. MMWR Recomm Rep 2023;72(No. RR-4):1–19. DOI: http://dx.doi.org/10.15585/mmwr.rr7204a1


